Instructions
This calculator interface is heavily based on flores' yanc (Yet Another Nutrient Calculator)
but implements some extra features and is based on different data.
This tool is used to do some different calculations on fertilization.
Some therms need to be defined to understand how it works.
An important WARNING: a wrong fertilizers' dose may lead to death fish,
invertebrates and plants. We can't ensure that all results are right, so, you
are strongly encouraged to check results with other tools. Please report to us
any wrong result or bug so we can improve this tool reliability.

- Dose to reach target: How much fertilizer you need to add to tank to reach the target value (in ppm) of a specific element
- The result of a dose: How much of each element of the compound will be added to tank with a given dose.
- Estimative Index: The same as (1) but the target is the value expected from E.I. Protocol
- Estimative Index Daily: The same as (3) but for daily dosing
- Estimative Index Weekly: The same as (3) but for weekly dosing
- Perpetual Preservation System: The same as (1) but the target is the value expected from P.P.S. Protocol
- Poor Man Dosing Drops: The same as 1) but the target is the value expected from P.M.D.D. Protocol
The calculations are operated over diy (do-it-yourself) products that can be powder or solutions of basic elements (or compounds): You can use them as pure powder [or solution (as-is) if they born as solutions] or add water and/or mix other elements to obtain your own diy solution.
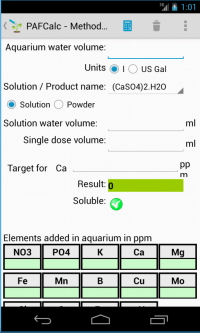 Parameters involved in operation are:
Parameters involved in operation are:
- The tank water volume: this is the net volume of water contained in the aquarium (filter volume should be excluded). You can chose in Units if value is in liters or US gallon.
- The diy initial compound.
- Then you must chose if you are dosing a solution or powder. In fact it may happen that the amount of the powdered compound, to be dosed into the aquarium, is too small to be measured (usually the case) and it may be more convenient to prepare a solution of water and compound. If you chose solution 2 extra values are required:
- The solution water volume: This is the amount of water (in ml) used to solute the compound
- Dose volume: The dose of solution (in ml) that you will add to tank. It should be a comfortable dose not to little that you cant measure it, not so big that you can't use.
- At this point you should chose the method alias the operation (1 to 7 of previous list) you want to calculate. Depending on method following informations may or not be required.
- The target element - that is the primary element you add to the water of aquarium when you add the compound (secondary elements qty are also showed in the Elements Added table). For method (1) you should add the value in ppm (see *) you want to add. In method 3 to 7 this value is dependent from method and is showed after calculation. In method (2) this value is not required.
When you use method (2) you are requiring to calculate the result of your dose so there is an extra field (Adding) where you should indicate this value and chose the unit (mg/ml or g). In all other calculations no more data are required so you can press Calc button.
The results:
For method (2) the values on the Elements Added table are the amounts of each
element you are adding in aquarium with that dose.
For other methods the Result field show the value of compound or solution you
should add in aquarium to reach the target that you choose or the target that
is defined by the method you choose.
An appropriate icon indicates whether the solution is soluble in water or less.
In case of indication of insolubility you must increase the dilution or reduce
the dose (depending on the case).
Specific alerts (depending from primary element) can also appear to show if
toxicity thresholds are exceeded or if the compound has any contraindications.
* ppm: In science and engineering, the parts-per notation is a set of pseudo units to describe small values of miscellaneous dimensionless quantities, e.g. mole fraction or mass fraction. Since these fractions are quantity-per-quantity measures, they are pure numbers with no associated units of measurement. Commonly used is ppm (parts-per-million, 10–6). In case of water solutions being 1l of pure water = 1kg it follows that 1ppm=1mg/l.
